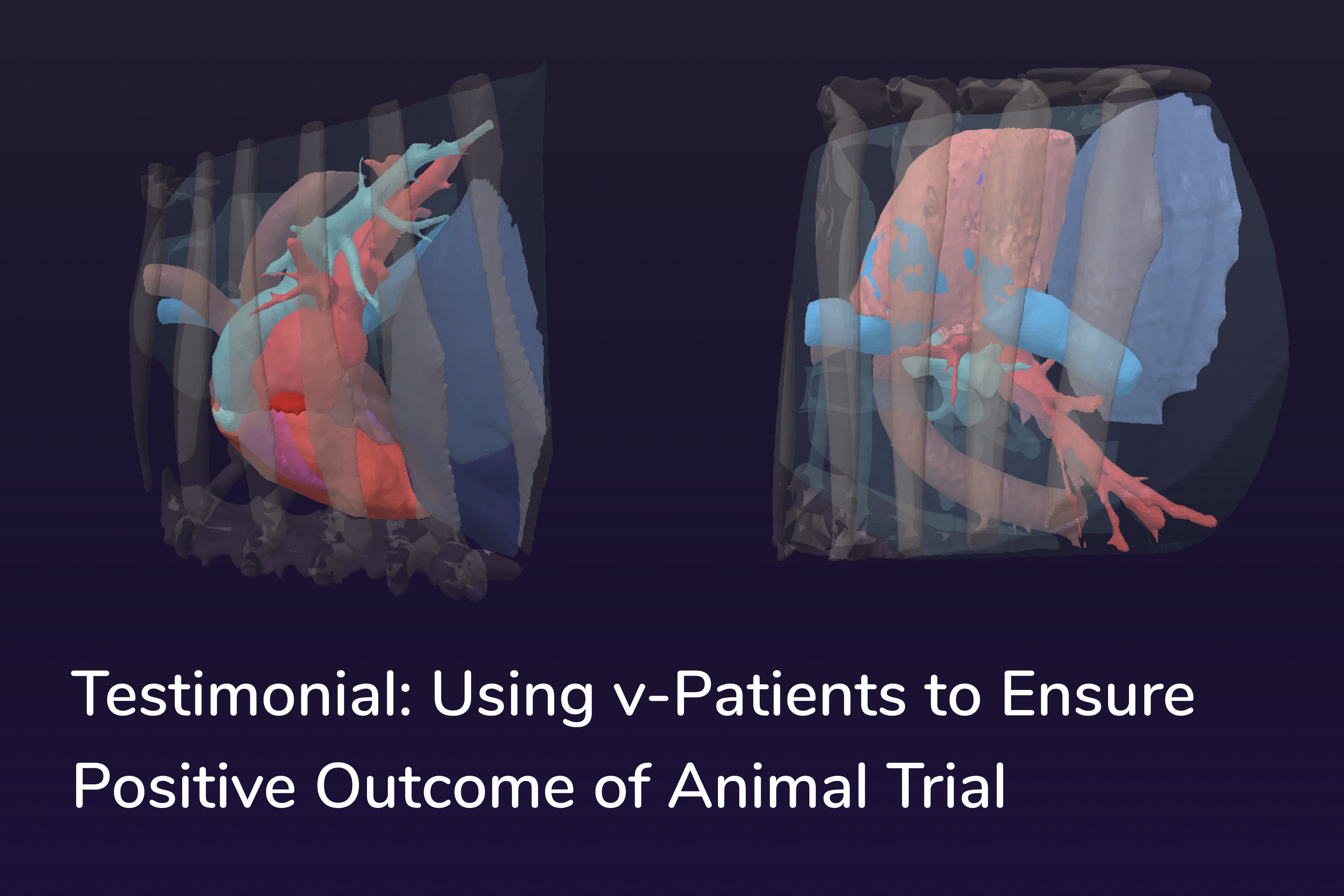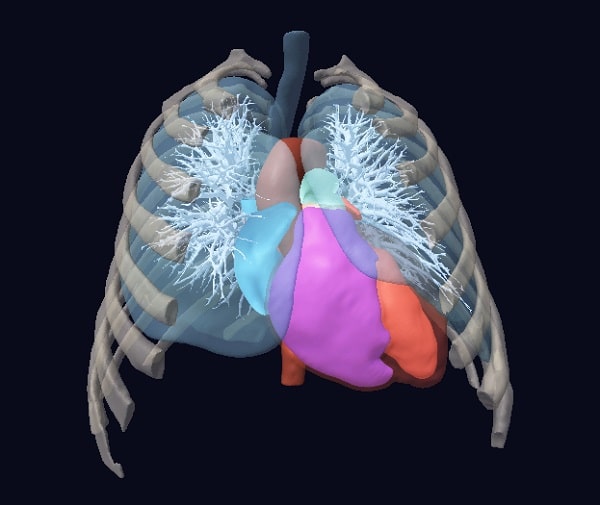
Accelerating and de-risking medical device development with digital patient twins
Virtonomy introduces v-Patients, enabling medical device developers to perform development and testing in a virtual environment, thereby accelerating development, reducing risks, expenses, and regulatory burden. Our end-to-end digital twin and simulation solution is based on an ever-expanding database of real clinical data to reflect anatomical variability, demographic diversity and pathological conditions.
 Streamline development
Streamline development
Optimize your device using advanced anatomical studies and simulations and shorten the iteration loop from years to weeks
 Significantly reduce costs and the risk to fail
Significantly reduce costs and the risk to fail
Test early for a comparably low price in (virtual) humans and ensure product safety & performance prior to and after clinical trials
 Ensure clinical trial success
Ensure clinical trial success
Perform anatomical analysis and simulations for many patients to ensure the optimal design and maximize population coverage
Read more in a blog post

Use virtual patients as digital evidence for submission to regulatory bodies
Regulatory viewpoint
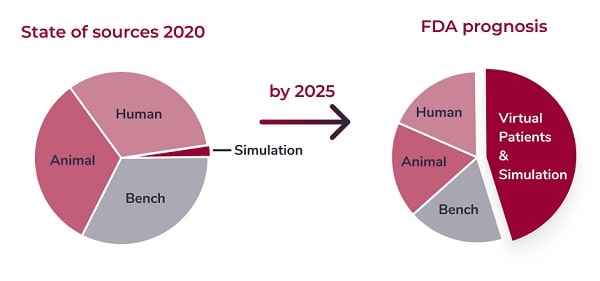
The FDA and European Commission show strong support for virtual patients and simulation.
Useful links:
FDA’s support on computational modeling
FDA: Diverse racial and ethnic groups for clinical trials
EU Commission: Recognizing benefits of computational modeling
EU Directive: Replace all animal research via computer simulations

FDA’s Office of Science and Engineering Laboratories (OSEL) has committed significant resources for transforming computational modeling from a valuable scientific tool to a valuable regulatory tool because of its potential for significant cost-savings in evaluating medical devices, simulating performance under scenarios that may not be possible with human use or that could more effectively be evaluated with simulation.


Want us to help you draft a virtual testing plan?
Ask our regulatory experts to establish your virtual testing plan connected to your physical testing. We provide this service for free during a special workshop tailored to you. Learn about the new FDA guidance documents and ASME standards for Computational Modeling and Simulation. Our experts will highlight how you can apply them and streamline your regulatory process.

Understanding patient anatomies and optimizing anatomical fit directly impacts the fate of development projects. Virtonomy’s combined expertise in specific pathologies, device/patient interactions and state of the art therapy options is tremendously valuable for anyone looking to create or optimize cardiovascular technologies.
Dr. Maximilian Kütting, Director R&D New Valve Technology

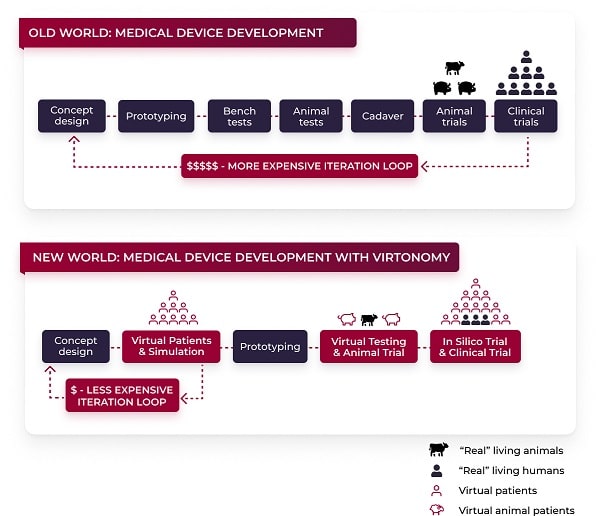
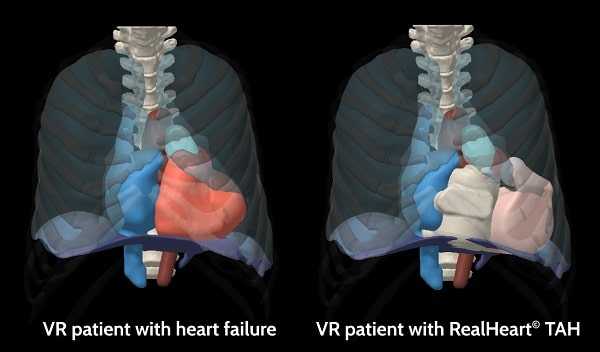
Our end-to-end digital twin solution
Virtonomy’s virtual trial platform supports the full product life cycle of medical device development, from concept phase to regulatory reporting and clinical practice
Thousands of Virtual Patients
- Virtual patient cohort tailored for you
- Advanced 3D visualization and interactive implantation
- Accurate 3D measurements and extensive statistics
Advanced Statistical Shape Models
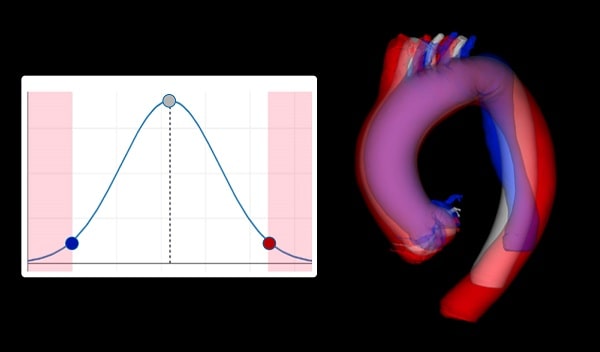
- Worst and edge-case anatomies
- Average patient anatomy
- Available for any customer-chosen patient group
Advanced Anatomical Simulations
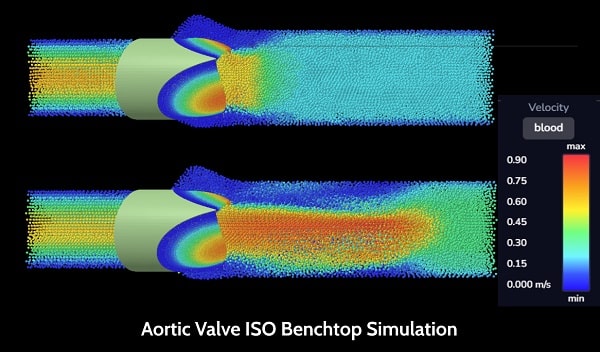
- Validated ISO benchtop test simulations
- Validated in-human implant simulations
- You control the simulations, we take care of all difficulties
Regulatory Services & Consultation

- Regulatory path with a virtual testing plan
- Compliance with all regulatory bodies
- Validated virtual evidence report
Latest from our blog
-
Catheter Design: Elevating Performance with Virtual Patients and Medical Simulation
Introduction to Cardiac Catheterization and Its Importance Cardiac catheterization is a cornerstone of cardiovascular intervention, and the gold standard for minimally invasive access to the heart. It is, therefore, suitable for all kinds of applications such as stent placement in the cardiac system, valve replacement or treatment of ventricular fibrillation. … Catheter Design: Elevating Performance…

How can you shorten your time-to market?
Talk to our experts to find out how you can utilize digital twins for your specific medical device.
Our supporters and partners