
Accelerating and de-risking medical device development with digital patient twins & medical simulations
Virtonomy introduces v-Patients, enabling medical device developers to perform development and testing in a virtual environment, thereby accelerating development, reducing risks, expenses, and regulatory burden. Our end-to-end digital twin and simulation solution is based on an ever-expanding database of real clinical data to reflect anatomical variability, demographic diversity and pathological conditions.

Trusted by

We cover a wide range of medical devices
Heart valves
Anticipate anatomical and positional changes during the cardiac cycle, significantly reducing the risk of implant failure.

TAH and Heart Assist Devices
Simulate blood flow dynamics, anatomical variations, and long-term device performance, ensuring optimal fit and function.

Occluders
Pre-test device deployment, positioning, and stability, ensuring effective closure and minimizing risks of migration or residual shunts.

Catheters
Simulate navigation through complex vascular pathways and assess catheter positioning, stability, and functionality in patient-specific anatomies.

Cannulas
Simulate insertion and flow dynamics within patient-specific vascular or cardiac anatomy, which enhances precision in cannula sizing, placement, and functionality for critical care.

Stents
Virtually test deployment, expansion, and arterial wall interaction in individualized vascular models.



Our end-to-end digital twin solution
Virtonomy’s virtual trial platform supports the full product life cycle of medical device development, from concept phase to regulatory reporting and clinical practice
Thousands of Virtual Patients
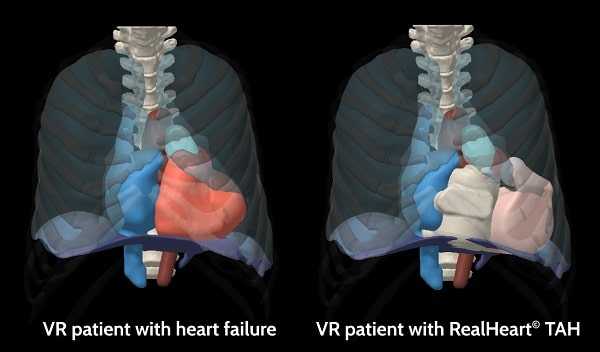
- Virtual patient cohort tailored for you
- Advanced 3D visualization and interactive implantation
- Accurate 3D measurements and extensive statistics
Advanced Statistical Shape Models
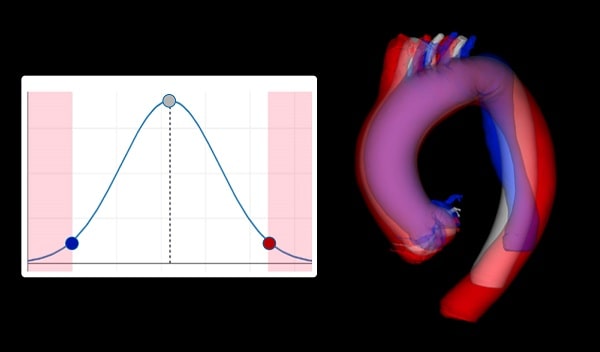
- Worst and edge-case anatomies
- Average patient anatomy
- Available for any customer-chosen patient group
Advanced Anatomical Simulations
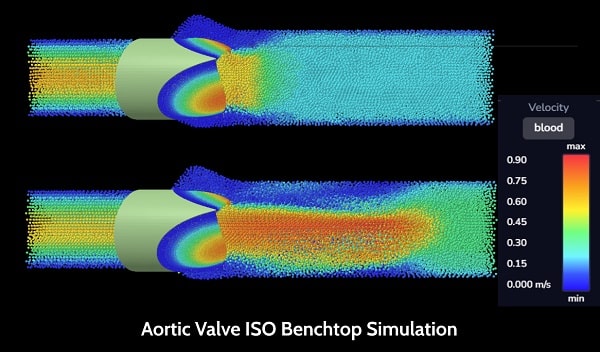
- Validated ISO benchtop test simulations
- Validated in-human implant simulations
- You control the simulations, we take care of all difficulties
Regulatory Services & Consultation

- Regulatory path with a virtual testing plan
- Compliance with all regulatory bodies
- Validated virtual evidence report
Use our software in any stage of your development – from early design concept up to clinical use.

Advanced Simulations
Beyond virtual patients, we offer comprehensive simulation services to validate device performance and optimize designs.
Fluid dynamics
Blood flow analysis, pressure mapping, and hemodynamic studies for cardiovascular devices.
Structural Mechanics
Stress analysis, fatigue testing, and material deformation for implant durability assessment.
Device Deployment
Catheter navigation, stent expansion, and implant positioning in patient-specific anatomies.
Performance Modelling
Long-term device behavior, wear patterns, and efficacy predictions across patient populations.
- Regulatory Compliance – approved by FDA and EU Regulatory Bodies as digital evidence
- ISO Compliant
- ASME V&V40 – Validated Methods

Benefits of using digital twins

Streamline development
Optimize your device using advanced anatomical studies and simulations and shorten the iteration loop from years to weeks.

Reduce costs and the risk to fail
Test early for a comparably low price in (virtual) humans and ensure product safety & performance prior to and after clinical trials

Ensure clinical trial success
Perform anatomical analysis and simulations for many patients to ensure the optimal design and maximize population coverage



Understanding patient anatomies and optimizing anatomical fit directly impacts the fate of development projects. Virtonomy’s combined expertise in specific pathologies, device/patient interactions and state of the art therapy options is tremendously valuable for anyone looking to create or optimize cardiovascular technologies.

Dr. Maximilian Kütting
Director R&D New Valve Technology
Use virtual patients as digital evidence for submission to regulatory bodies
The FDA and European Commission show strong support for virtual patients and simulation.

FDA’s Office of Science and Engineering Laboratories (OSEL) has committed significant resources for transforming computational modeling from a valuable scientific tool to a valuable regulatory tool because of its potential for significant cost-savings in evaluating medical devices, simulating performance under scenarios that may not be possible with human use or that could more effectively be evaluated with simulation.


Want us to help you draft a virtual testing plan?
Ask our regulatory experts to establish your virtual testing plan connected to your physical testing. We provide this service for free during a special workshop tailored to you. Learn about the new FDA guidance documents and ASME standards for Computational Modeling and Simulation. Our experts will highlight how you can apply them and streamline your regulatory process.
Latest from our blog
-
What Does the FDA Think About In Silico Evidence?
One of the most common questions we get and a question that keeps coming up in cardiovascular device development circles, in regulatory affairs meetings, and in conversations between engineers who have just run their first digital twin simulation is “Will the FDA actually accept this?” It’s a fair question. And
-
Accelerating In Silico Medical Innovation: Virtonomy Transforms Healthcare Simulation enabled by NVIDIA
The medical device industry faces many challenges and pressures to reduce development timelines, cut costs and navigate complex regulatory pathways. Traditional development approaches rely heavily on animal testing and extensive clinical trials – processes that can take years to complete and many iteration loops due to failed trials. With new,

See our platform
v-Patients in action
Get a demo and see how our customers use v-Patients to speed up their device development and save costs.













