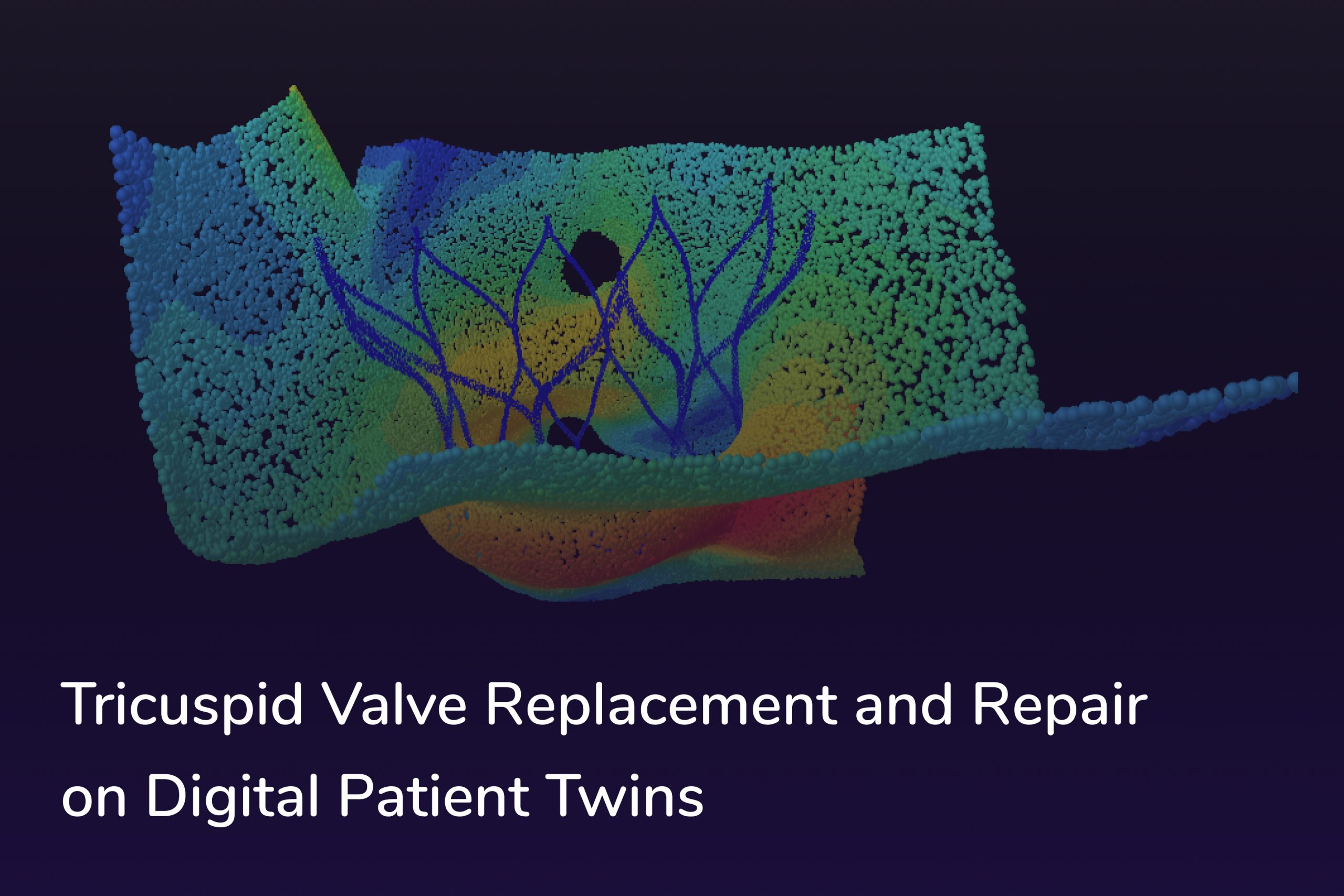
Tricuspid regurgitation, characterized by the backward flow of blood from the right ventricle into the right atrium due to a malfunctioning tricuspid valve, is a condition that can cause a cascade of health issues if left untreated. The challenges in diagnosing and managing this condition often stem from its complex, subtle nature and anatomical differences among patients. The development of our innovative digital twin technology now assists in understanding these variances better, enabling more precise interventions and safer tricuspid valve replacement procedures.
Tricuspid regurgitation necessitates the development of a transcatheter solution that is simple, safe, and reliable. Implant developers must design a percutaneous replacement system that is easy to deploy and functional. It is essential for such a device to be flexible and adaptable to accommodate inter-patient variability and ensure long-term regulation of blood flow. Furthermore, implant developers must ensure that anatomical and positional variations during the intra-cardiac cycle do not result in implant failure. With the aid of digital patient twins and advanced medical simulation tools, implant developers are now better equipped to anticipate anatomical and positional changes during the cardiac cycle, significantly reducing the risk of implant failure. This approach offers a more accurate, personalized, and cost-effective strategy for tackling the complexities associated with tricuspid regurgitation.
Challenges in treating TR (Tricuspid Regurgitation) and how we can address them with digital patient twins and simulations
| Challenges in treating TR | How can we address them? |
|---|---|
| Ensuring Device Effectiveness: Correct size, design, positioning, and material selection of the device are crucial for effectiveness | Perform virtual implantation of various device versions on a large number of virtual TR patients |
| Complex and unique shape of Annulus Structure: The unique anatomy of the tricuspid valve, which changes shape significantly throughout the cardiac cycle, presents challenges for repairing and anchoring replacement devices. | Design and optimize innovative repair and anchoring mechanisms tailored to the target patient cohort |
| Patient Selection Criteria: Patient selection based on the severity of TR is a pivotal factor in device testing and justifying intervention | Investigate specific anatomical measurements for a statistical population analysis to explore worst-case scenarios, establish inclusion/exclusion criteria, and validate patient selection protocols |
| Dynamic Loading: Devices experience dynamic periodic loading, increasing the risk of material failure. | Conduct fatigue analysis using simulations taking into account many parameter combinations |
| High Resolution Costs: Traditional test methods, including tensile tests, fatigue evaluation, and flow assessments, are performed after prototyping, leading to high resolution costs for identified issues | Easily set up and execute simulations to evaluate the safety and performance of your device prior to creating physical prototypes. Perform simulations of anatomical conditions, including blood-organ interactions, ensuring compliance with ISO standard test cases. |
We have the data you need.
Our ever-expanding database currently consists of over 130 Tricuspid Valve Regurgitation Patients. Most of the patients are those of severe (torrential) regurgitation volume. The following figures show the distribution of severity:

Based on the CT scans, we 3D reconstruct the patient’s Right Atrium (RA) including IVC and SVC, Right Ventricle (RV), and Pulmonary Valve (PV) – for both end-systole and end-diastole.
For determining the most appropriate valve size, it is particularly important to have access to precise and validated measurements of specific anatomical structures, such as the Tricuspid Annulus Plane (TAP). Alongside our comprehensive database of three-dimensional models, we provide these measurements, including parallel cross-sectional slices, as well as the maximum and minimum cross-sectional diameters at both end-systole (ES) and end-diastole (ED) phases.

4 steps to a non-invasive validation of a valve

1. Define your desired patient cohort
Begin by accessing our extensive database, which includes over 130 3D models of the right heart. All models in our database have been derived from a diverse set of CT scans, ensuring a comprehensive representation of various cardiac conditions, including tricuspid regurgitation. Users can filter and select models based on specific criteria such as age, severity of condition, and anatomical variations to define their desired patient cohort. This tailored selection helps to focus the development process on a well-defined target demographic.
2. Study the measurements, evaluate average and edge-cases
Once the appropriate patient cohort has been selected, access detailed statistical population measurements based on your cohort selection from our database. These measurements provide valuable insights into dimensions, geometric variations, and other physiological characteristics related to the tricuspid valve and the anatomy of the right heart. This data can be leveraged to adapt your valve design’s sizing requirements to ensure optimal fit and performance across the selected patient population. Our software enables data-driven decision-making, allowing you to model and modify the device design to achieve the desired coverage of your target population, thus enhancing the potential market reach and clinical applicability of the valve.
3. Perform flow assessment and risk analysis
Within the software, perform advanced flow simulations combining your device and patient anatomies. These simulations are integral for evaluating the hemodynamic performance of the valve design under various conditions. You can choose to run these assessments on generic right heart models or customize them to include patient-specific anatomies based on your previously selected cohort. The software can simulate different flow rates and pressures to predict potential risks, such as hemolysis (damage to blood cells) or thrombosis (blood clotting). This step is critical for identifying and mitigating risks early in the design phase, ensuring the safety and efficacy of the valve. These simulations are compliant with DIN EN ISO 5840 standards.
4. Simulate valve implantation and durability
The final step involves simulating the actual implantation process of the tricuspid valve, or the tricuspid valve replacement. The simulation can be highly customized to a variety of scenarios which can include different surgical implantation approaches and the integration of additional delivery devices. Explore how different materials and designs react to the physiological environment of the heart, assess the ease of implantation, and predict the long-term durability and performance of the valve. This comprehensive simulation not only helps in refining the design but also provides critical insights into potential post-operative complications and the overall patient outcome.
How does a simulation of a valve fatigue testing work?
The traditional method of fatigue analysis is, in this case, replaced with a faster and much more cost-effective in silico method.
Computational methods for Valve Fatigue analysis:
- Calibration of DV material model criterion based on experimental investigation
- Dang Van (DV) based approach with the Lin–Taylor homogenization law for fatigue and material modeling
- Modified Goodman approach with resulting fatigue safety factor for a specific lifetime

And what about the blood?
Simulation results can be used to evaluate whether the device is functioning properly and analyze blood flow behavior after the tricuspid valve replacement surgery. You can examine the flow field characteristics to predict the risk of regurgitation, hemolysis, or thrombosis. With our simulation approach, it is possible to perform Flow Assessment compliant to DIN EN ISO 5840-1.
Want to know more?
Send us a message to schedule a free consultation with us, where we can discuss how to apply our digital twin technology to your use case and help you accelerate your clinical trials, calidate your device design and help you perform virtual fitting and virtual implantation on our large database of diverse virtual patients.