When One Size Doesn’t Fit All
Medical devices should work for everyone—not just for a narrow group of patients. True inclusive medical device design means creating solutions that fit people across age, sex, size, and anatomy. Yet too often, devices are designed and tested on limited datasets, which can lead to poor performance in real-world use.
Our software is built to change that. By combining simulation-driven tools with data-rich virtual patient models, we make it possible to test and optimize devices across diverse populations from the very beginning. This approach reduces risks, lowers costs, and ensures devices are designed with inclusivity in mind from day one. The following five features highlight how our platform supports inclusive design in MedTech.

1. Statistical Shape Models (SSMs): Designing for Anatomical Diversity
Statistical Shape Models represent a huge range of anatomical variability within a population, derived from large datasets of patient scans. Instead of relying on single cases, SSMs allow developers to systematically adjust parameters such as proportions, curvature, and size. This enables early-stage evaluation of device interaction with realistic anatomical differences.
Why it matters:
Design for anatomical variation in men, women, pediatric, or elderly patients without sourcing dozens of new datasets.
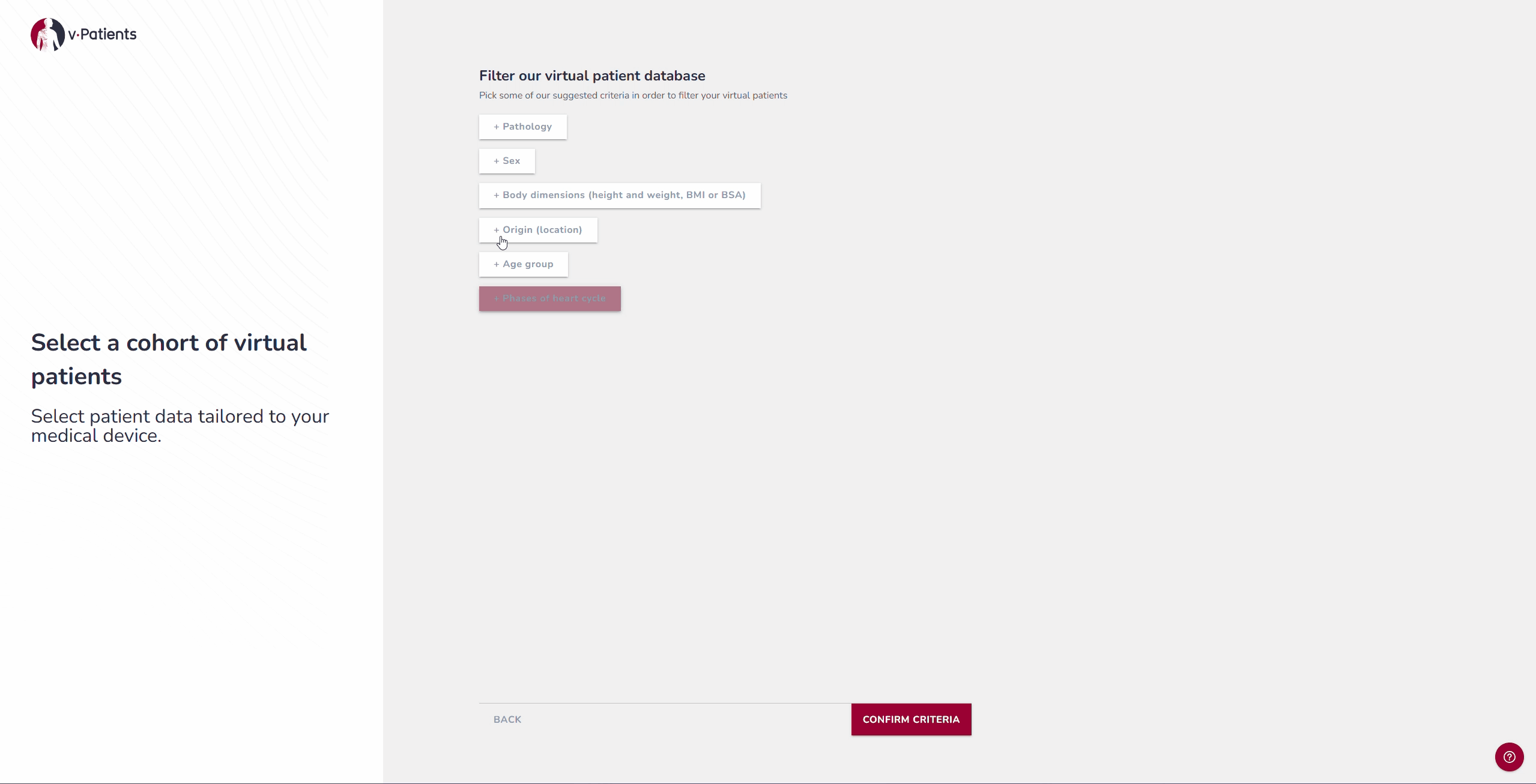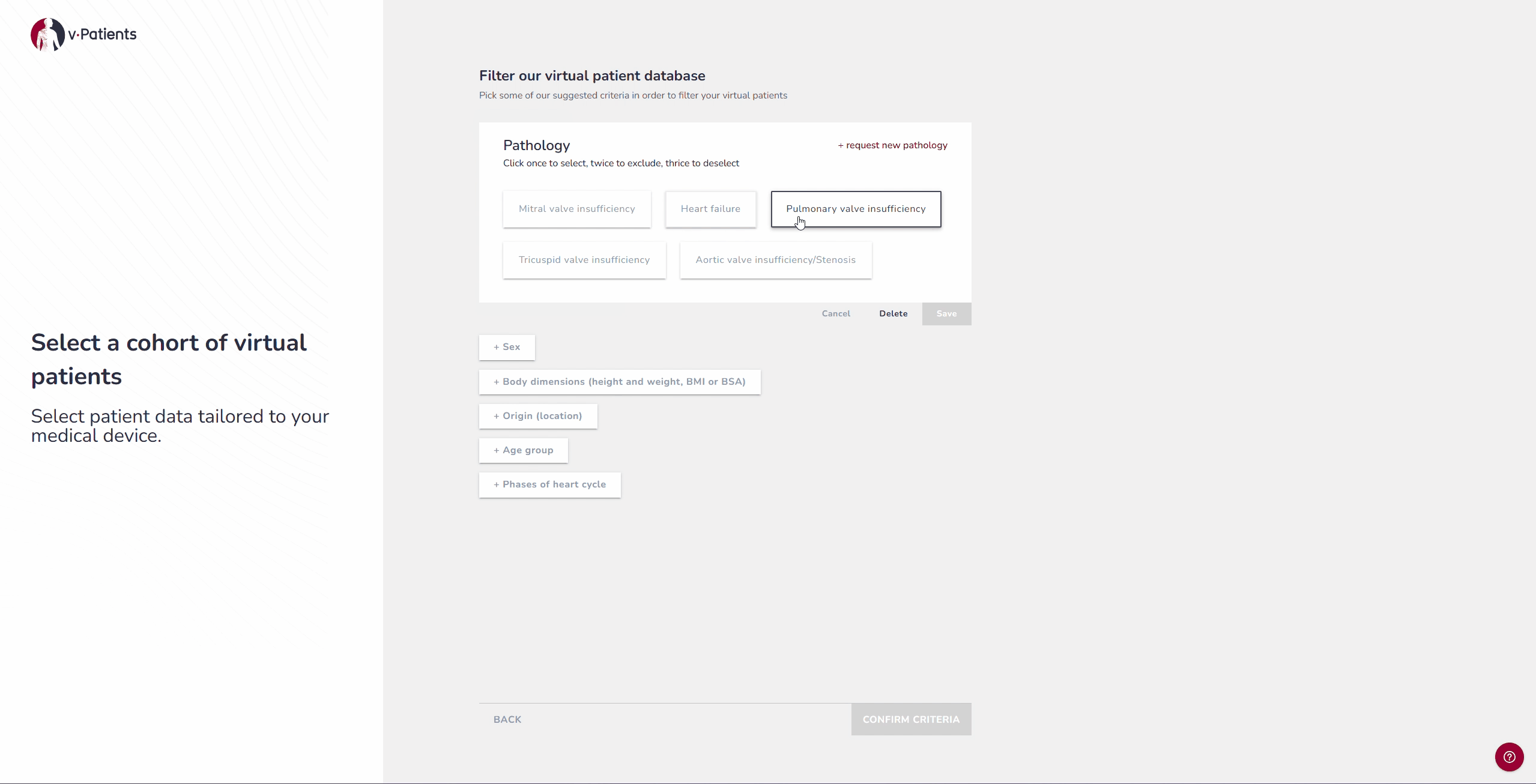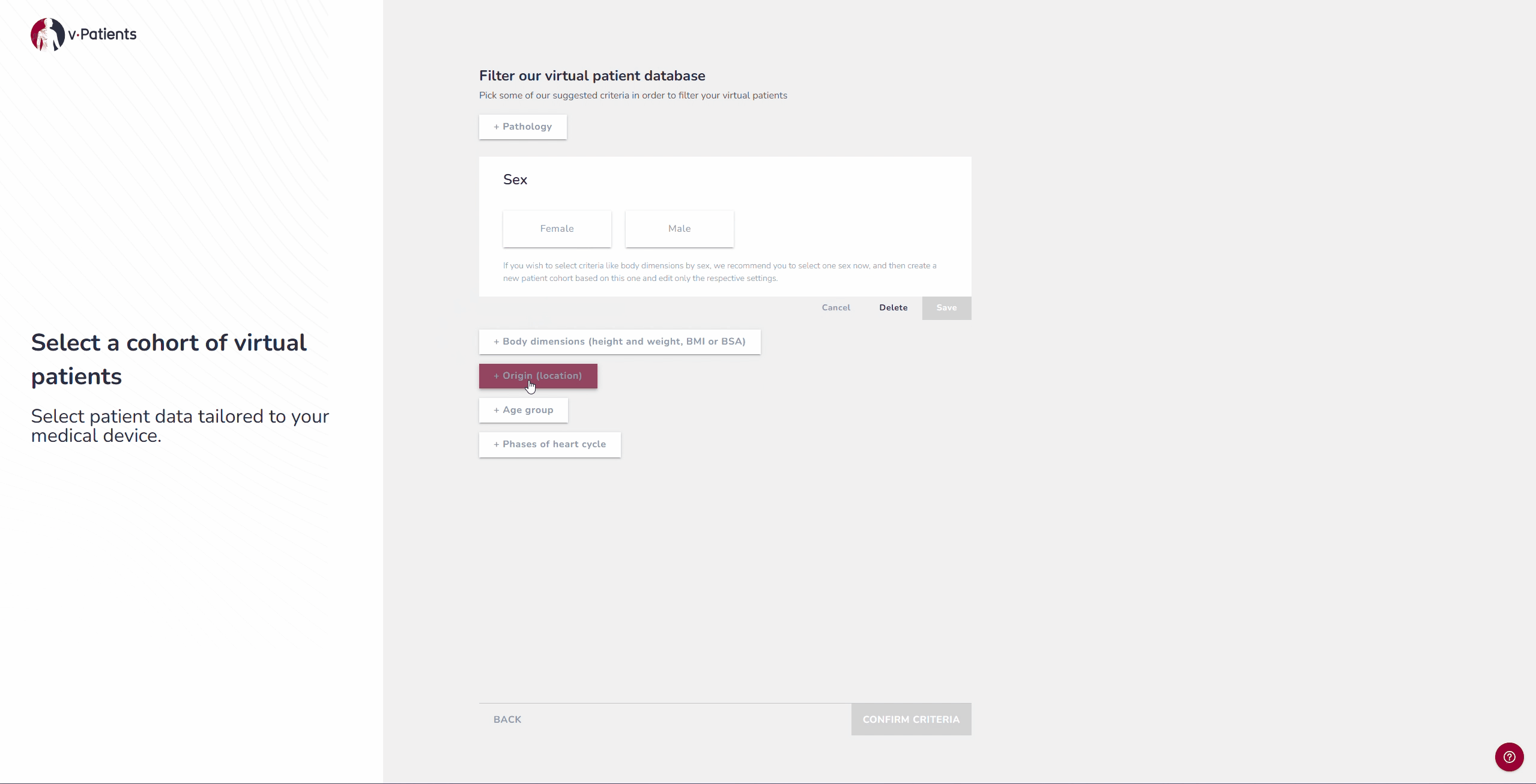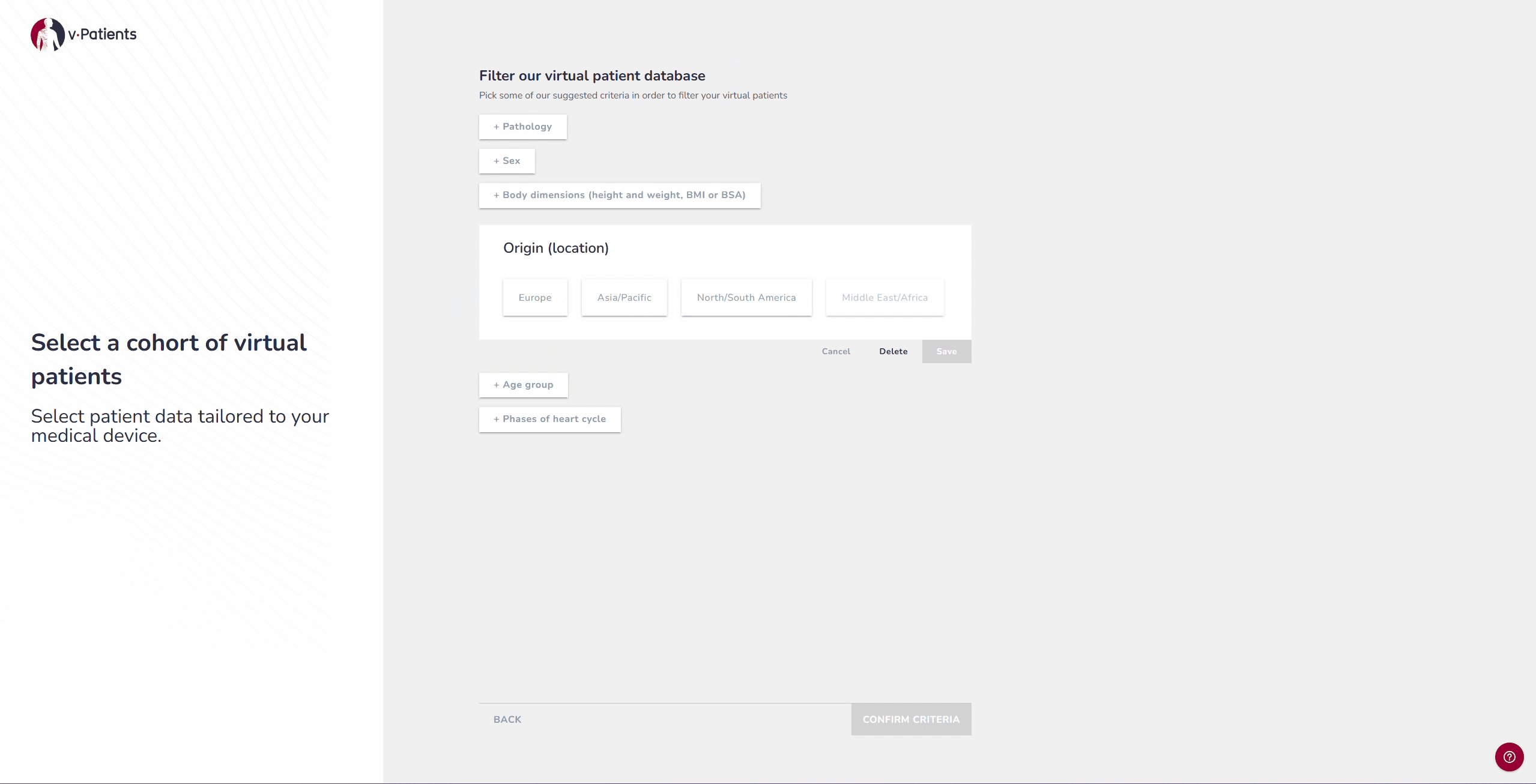
2. Virtual Patient Library: Representing Underrepresented Populations
Our digital patient twin library provides anatomies from a wide range of patient groups, including demographics that are often underrepresented in device design, such as women and children. Each dataset is segmented by our Data&AI Team and ready to be used in simulations, making it possible to include population diversity during early iterations rather than waiting until later testing phases. This helps ensure that devices are designed with inclusivity in mind from the start.
For example, if your device was tested on patients from the United States, it is possible to now test it on patients from Japan to explore the differences and compare the fit.
Why it matters:
Ensure your device works for everyone—not just the average male patient.
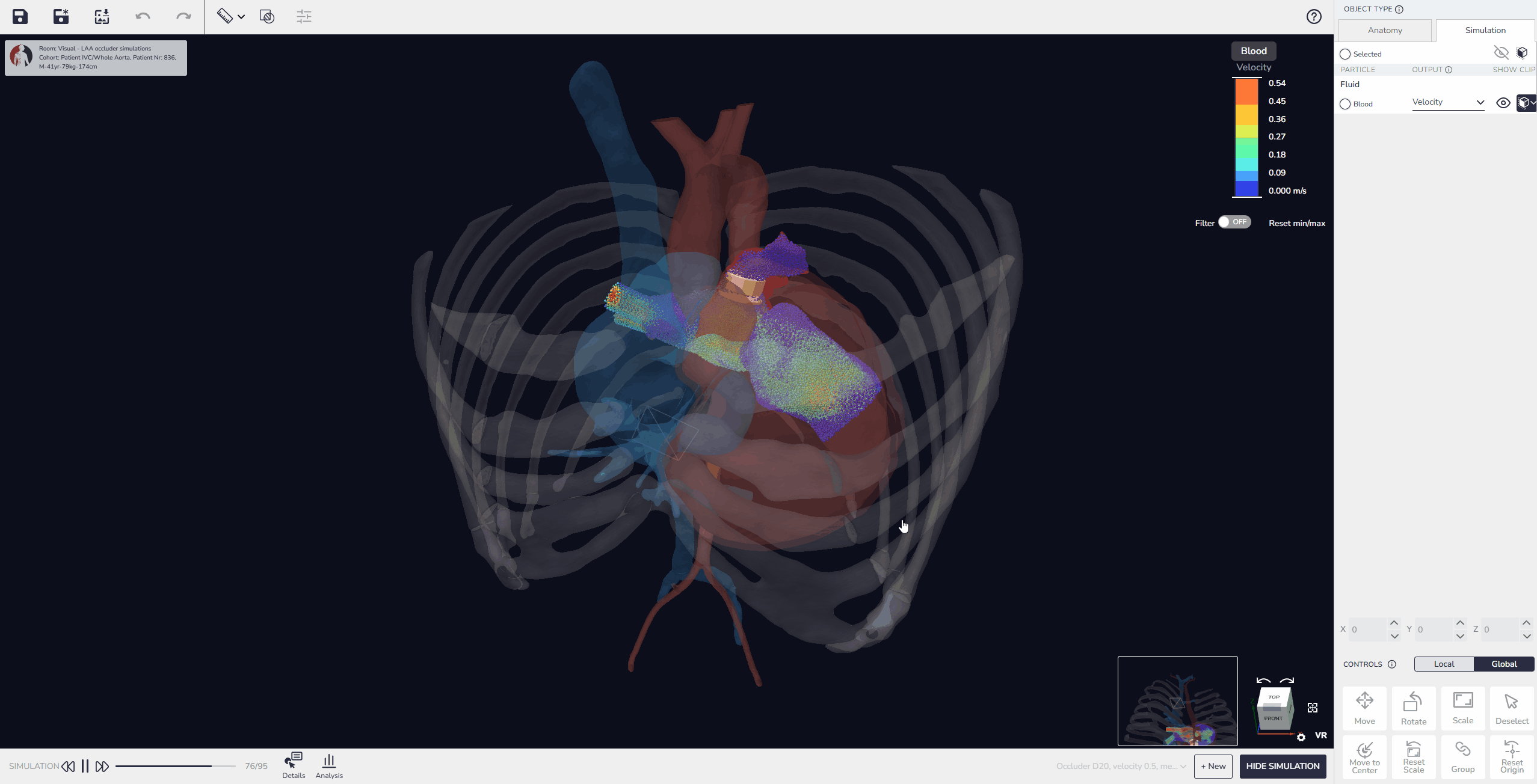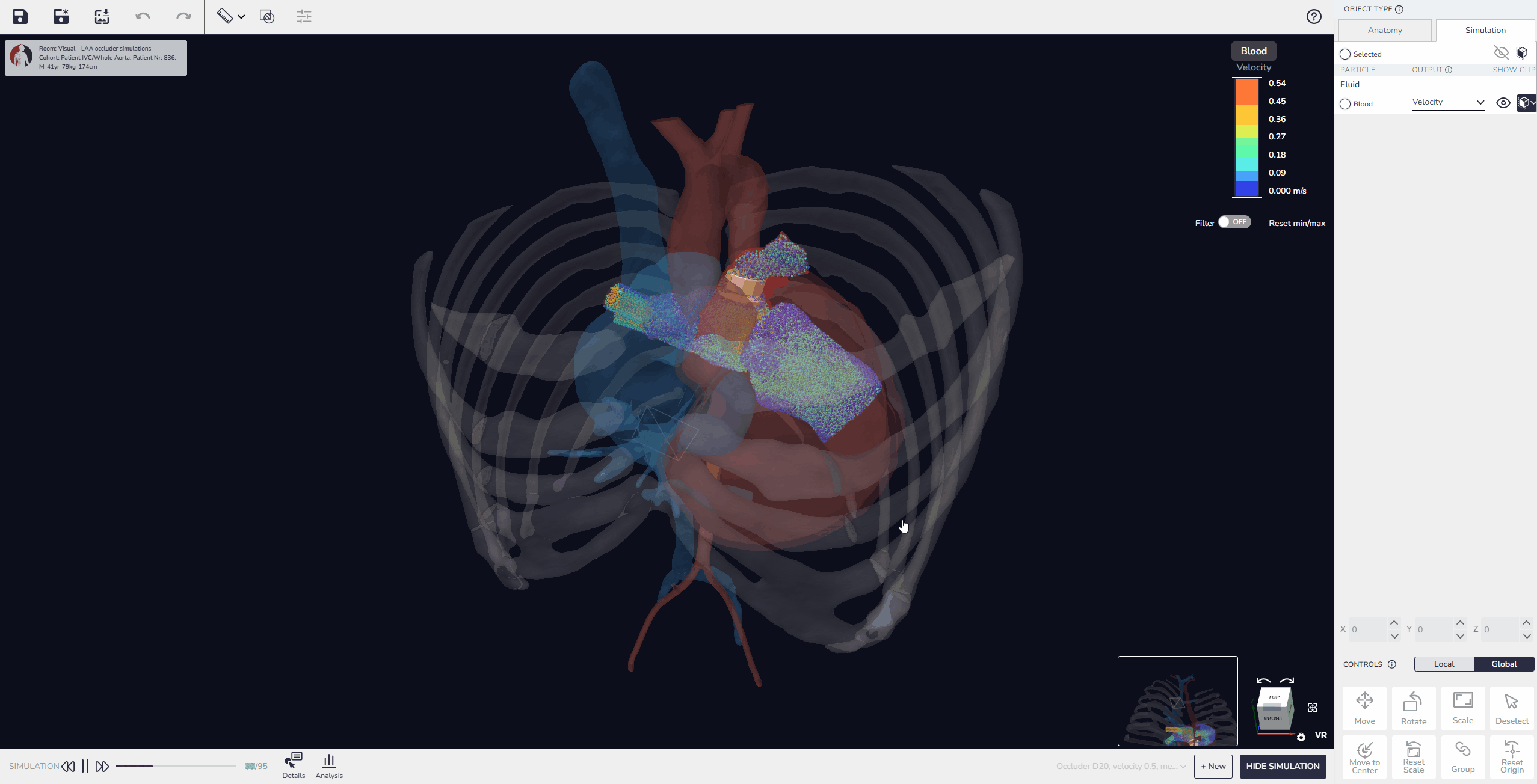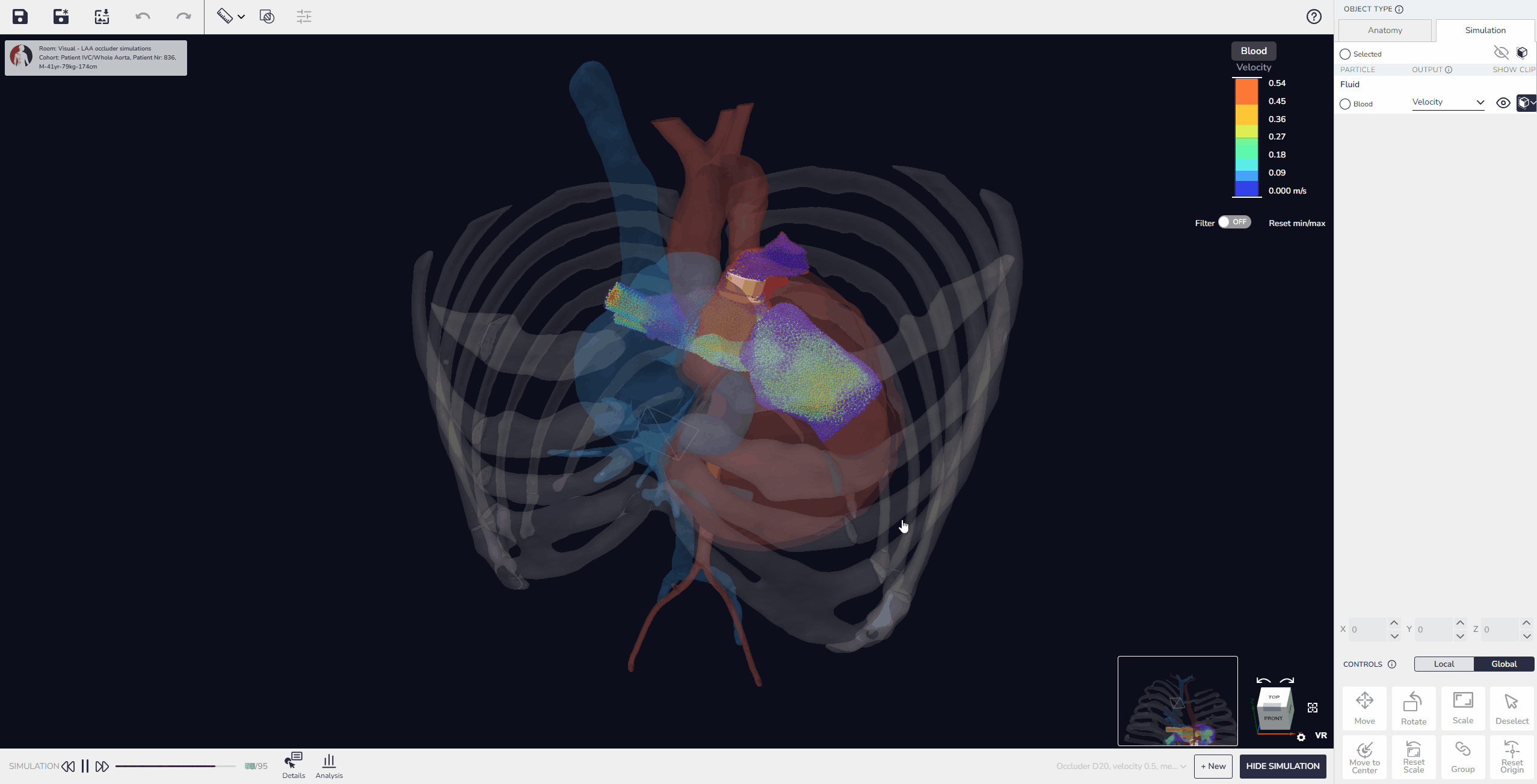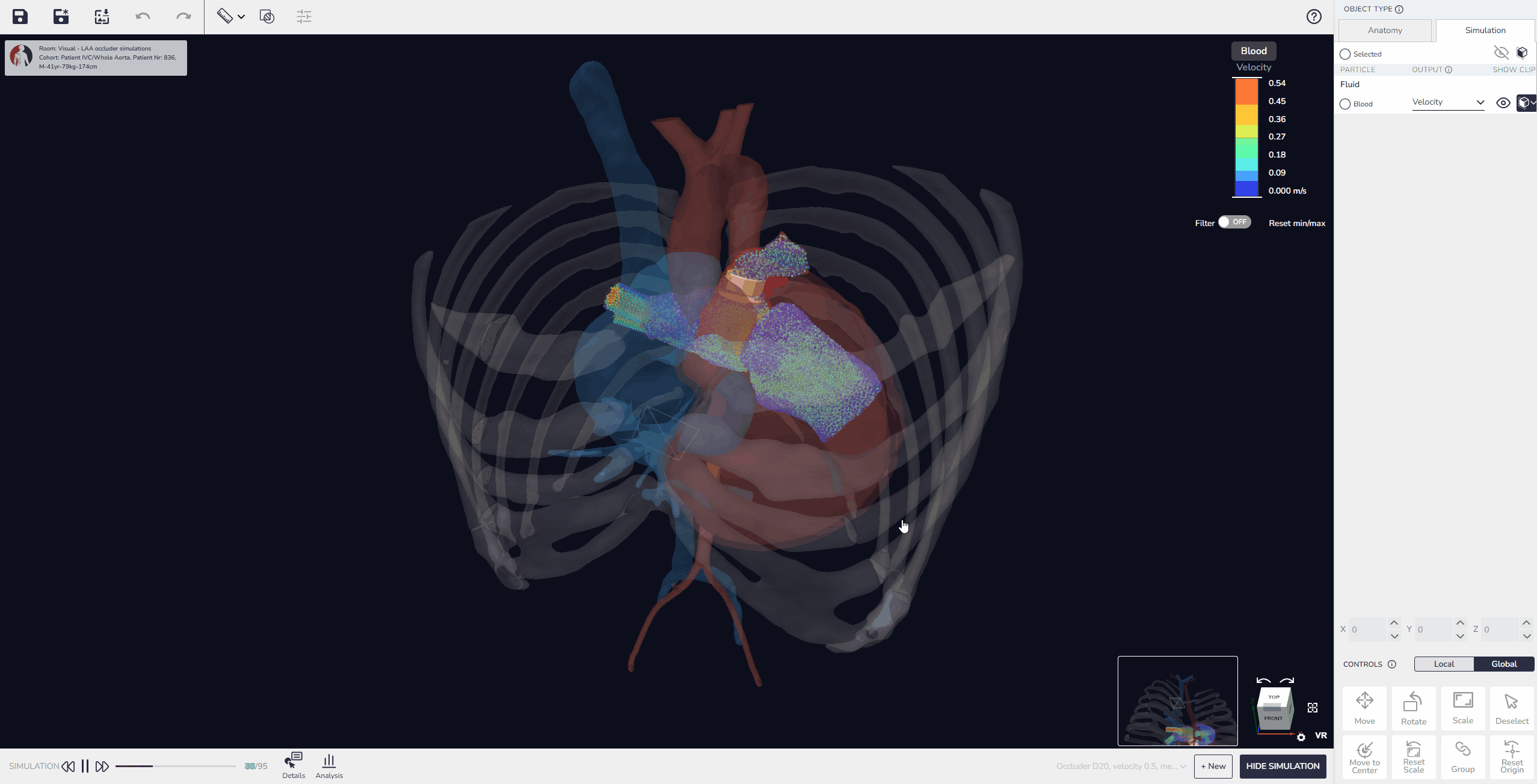
3. Virtual Implantation and Population Simulation: Testing Beyond the Average Patient
Our software allows developers to upload device models, position them within digital patient anatomies, and simulate placement and fit. Beyond single-patient testing, it is also possible to perform population simulations, running the same device design across multiple virtual patients to compare outcomes. These simulations can reveal patterns in performance, identify anatomical conditions that pose higher risks, and quantify variability in fit or alignment across the population.
This capability enables developers not only to test feasibility in individual cases but also to build comparative data that shows how a design performs in typical patients versus outliers. As a result, device performance can be optimized more effectively before advancing to physical prototyping or clinical evaluation.
Why it matters:
Instead of relying on isolated case studies, developers gain insight into how a device performs across a spectrum of real-world anatomies. This makes it possible to identify risks earlier, optimize designs more effectively, and build stronger evidence before moving to physical prototypes or clinical testing.
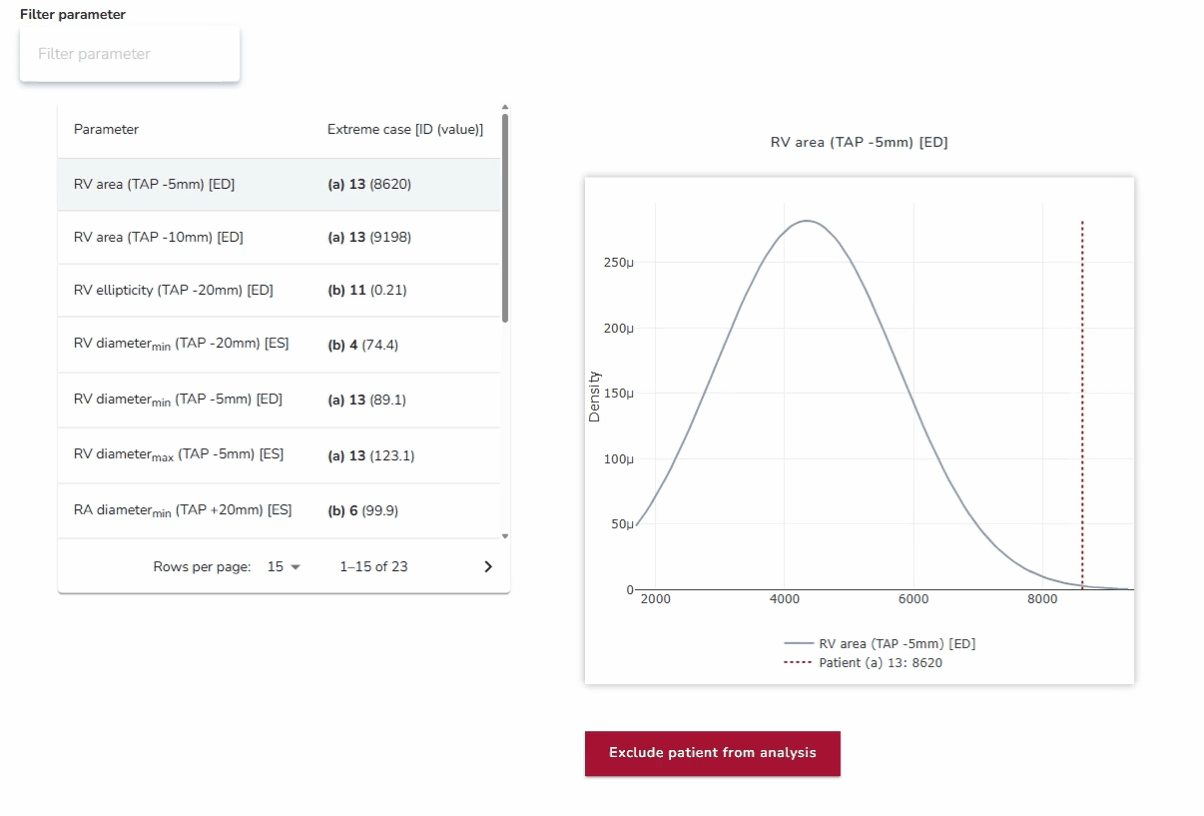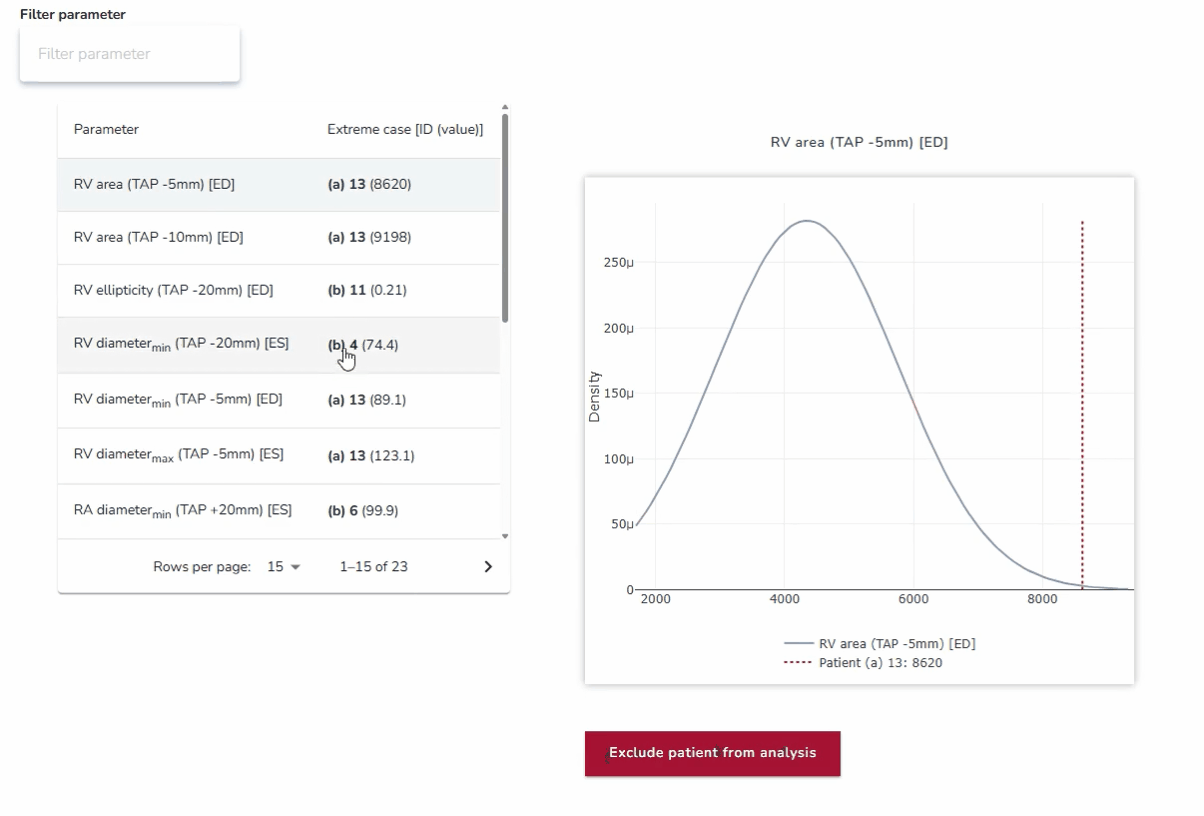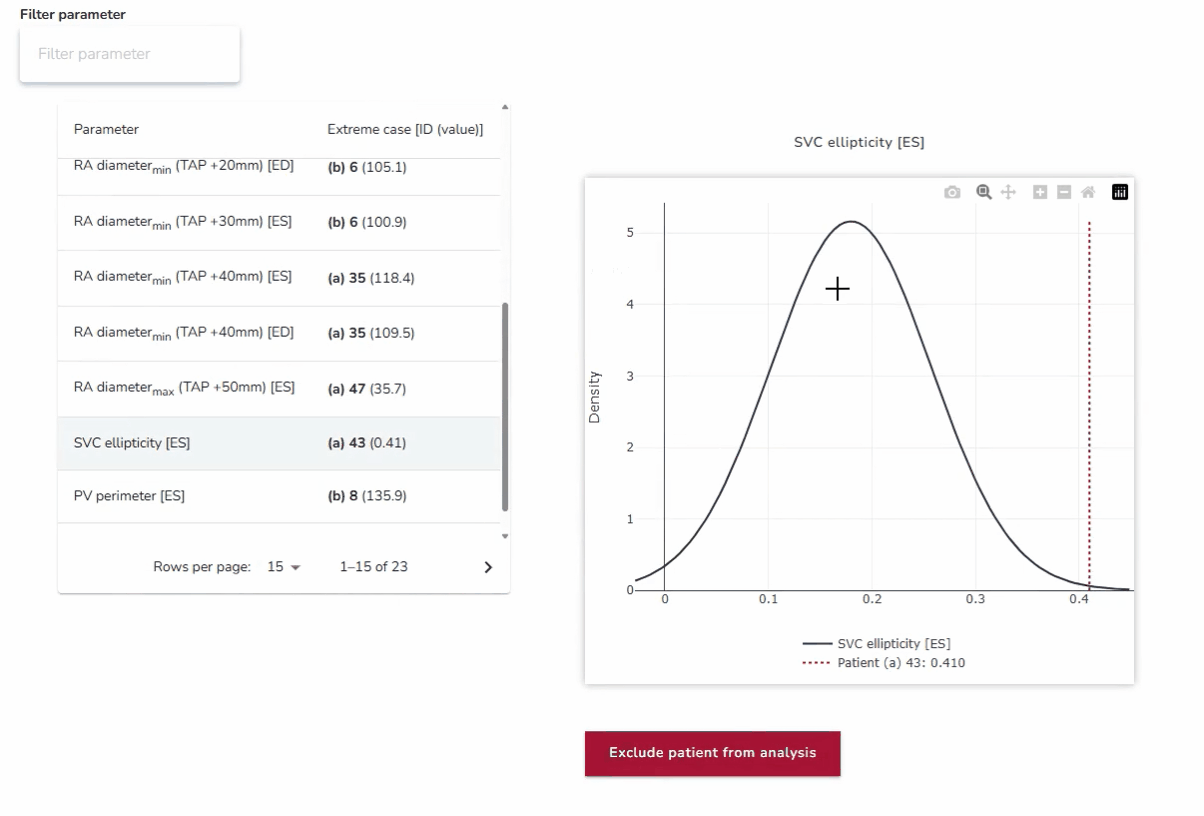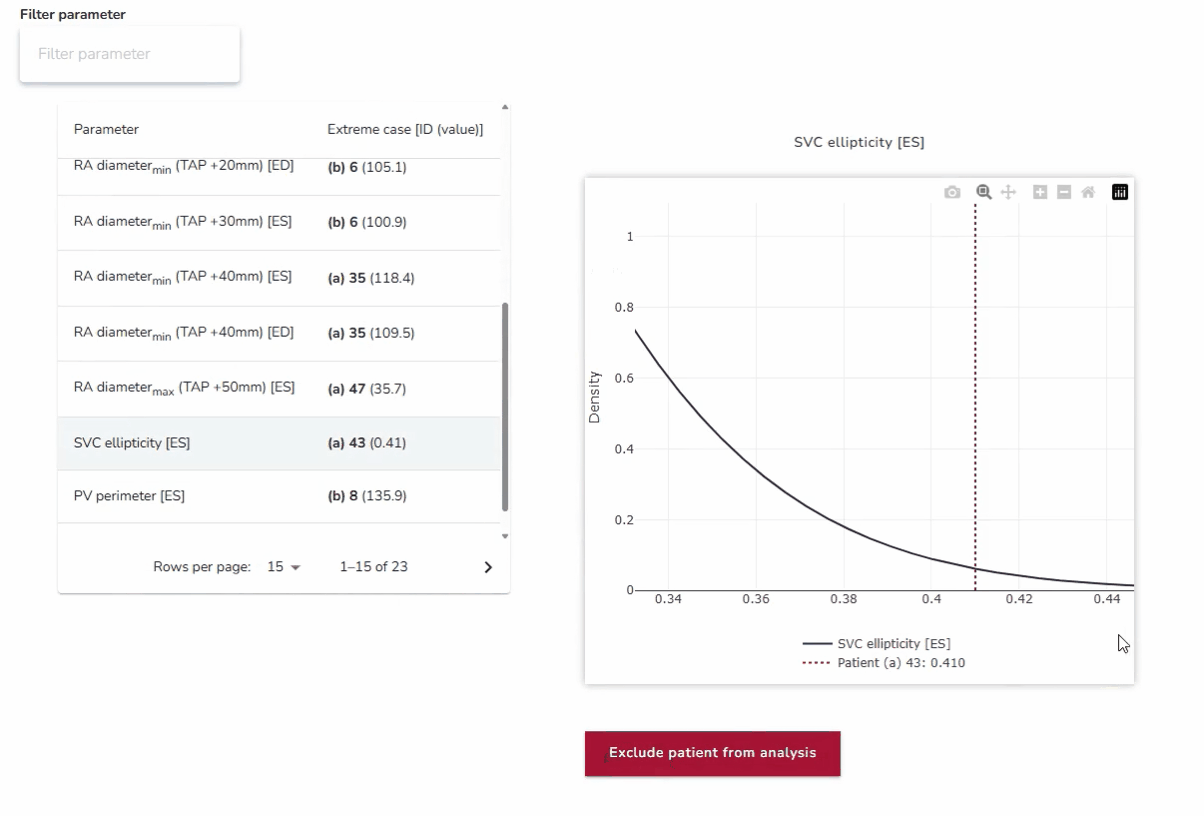
4. Best- and Worst-Case Scenario Testing: Ensuring Reliability Across Extremes
In addition to assessing average anatomies, the platform enables testing against anatomical outliers at both extremes. By analyzing how devices perform in best- and worst-case scenarios—such as unusually narrow or wide anatomical structures—developers can ensure robustness across the full spectrum of patient variability. This approach strengthens device reliability and addresses clinical needs beyond the average case.
Why it matters:
This approach ensures that devices are not only optimized for the average patient but also resilient in edge cases where the risks of poor performance or failure are highest.
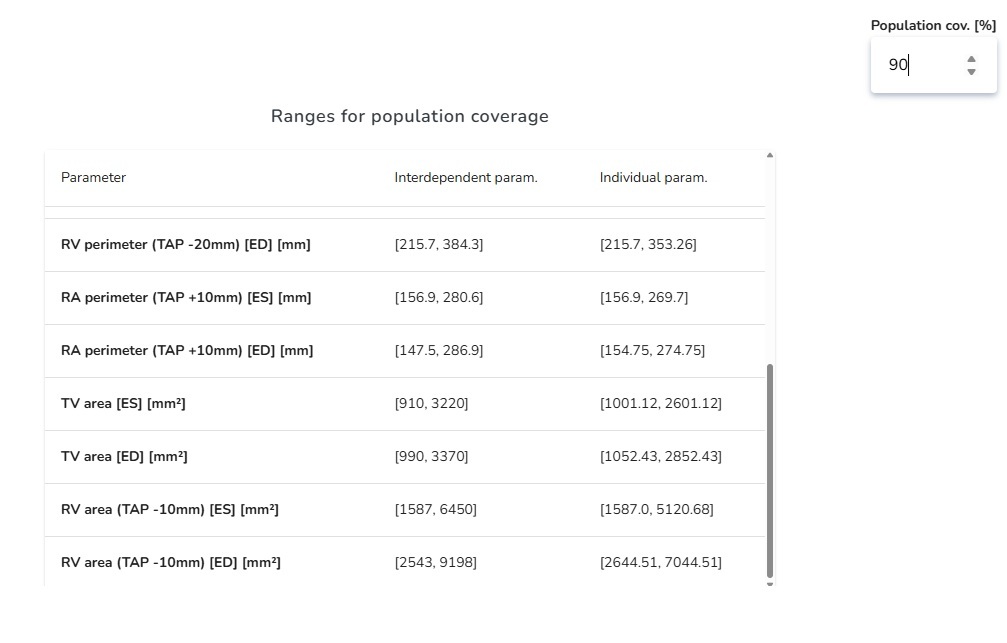
5. Population Coverage Analysis: Measuring Inclusivity in Device Design
Population Coverage Analysis allows developers to set a target—for example, designing a device that reliably covers 90% of the intended patient population. The software then calculates the anatomical parameter ranges (such as diameters, lengths, or curvatures) that must be addressed to achieve this level of coverage. Instead of guessing or relying on averages, developers get clear boundaries for what their design needs to accommodate.
For instance, if a vascular device is intended to cover 90% of patients, the software will specify the exact vessel diameter and length ranges required. This makes it possible to decide whether a single device design is sufficient or whether multiple size variants are necessary to achieve the desired coverage.
Why it matters:
By turning coverage goals into measurable design parameters, developers can make evidence-based decisions from the start. This not only helps ensure inclusivity and safety but also streamlines design iteration, reduces costly redesigns, and builds confidence that the device will succeed across a real-world patient population.
Inclusive Design as a Standard, Not an Afterthought
Achieving inclusive medical device design requires tools that reflect the full spectrum of human variability. By integrating statistical shape models, diverse patient data, virtual implantation and simulation, extreme-case testing, and population coverage analysis, our software provides a comprehensive framework for designing devices that serve more people, more effectively.
This approach helps medical device manufacturers move beyond average-case assumptions, ensuring safety, reliability, and inclusivity are built in from the start. The result: medical devices that are not only technically sound but also equitable, improving outcomes for patients everywhere.
To learn more about different use-cases, visit our page describing different usages here: See use-cases




