Pediatric cardiovascular medical device development faces a critical challenge: children are not just “small adults.” Their unique anatomies, rapid growth, and vulnerable physiology create hurdles that traditional research and testing methods struggle to overcome. Small patient populations, ethical limits on clinical trials, and high development costs leave most companies focusing on adult devices, while children—especially neonates and infants—remain underserved.
That’s why our recent webinar brought together leaders from CobiCure, Edwards Lifesciences, the FDA, and Virtonomy to explore how in-silico modeling and simulation can transform the pediatric device landscape. The full recording is available below, but here are the highlights.
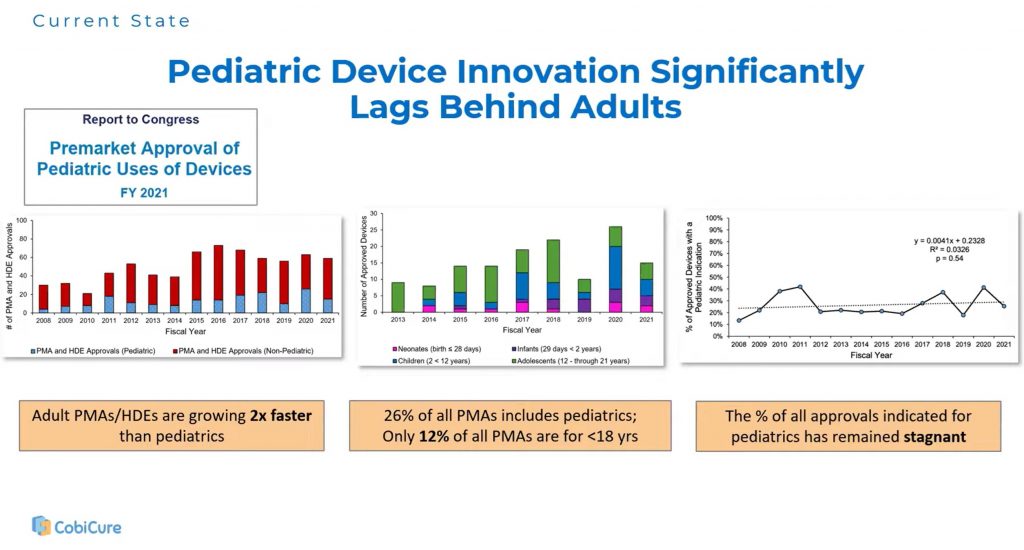
The Innovation Gap
Emma Moran, Head of MedTech at CobiCure, highlighted how far pediatrics has fallen behind. While adult device approvals continue to grow, pediatric approvals remain stagnant—most clustered in the 18–21 age range. For younger children, innovation lags significantly. Clinical studies are often underpowered, sometimes with fewer than fifty patients, and as a result, off-label device use in children is common but risky.
The economics make progress difficult: developing a novel therapeutic device can cost over half a billion dollars when capital and risk are included. With such small market sizes, the incentive for industry is limited. Emma argued that this is exactly where in-silico tools can shift the equation—reducing cost, accelerating learning, and making development viable.
How In-Silico Accelerates Development
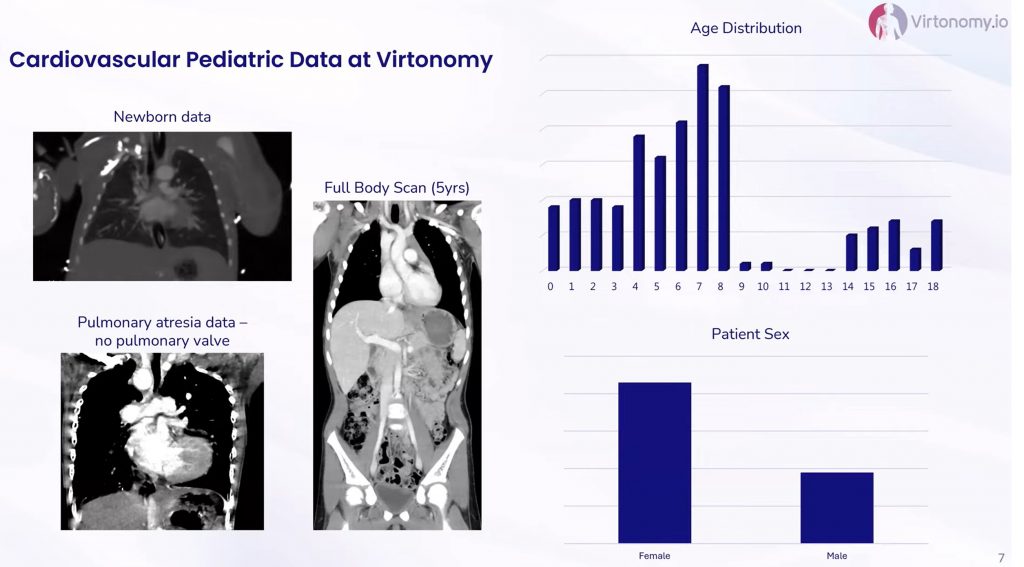
Virtonomy CEO Simon Sonntag walked through the practical workflow. Using large imaging datasets, Virtonomy creates virtual patients across pediatric age groups, from newborns through adolescence. These digital twins are then used for:
- Design optimization: testing devices against anatomical variability and identifying worst-case scenarios before physical prototypes are built.
- Trial planning: defining target cohorts, device sizing windows, and access routes to reduce patient burden and trial complexity.
- Procedural support: de-risking high-stakes procedures such as ECMO cannulation or pump implantation in tiny chests where space is limited.
By enabling early and repeated testing in a virtual environment, in-silico methods help teams learn faster, fail cheaper, and design safer.
The Regulatory Perspective
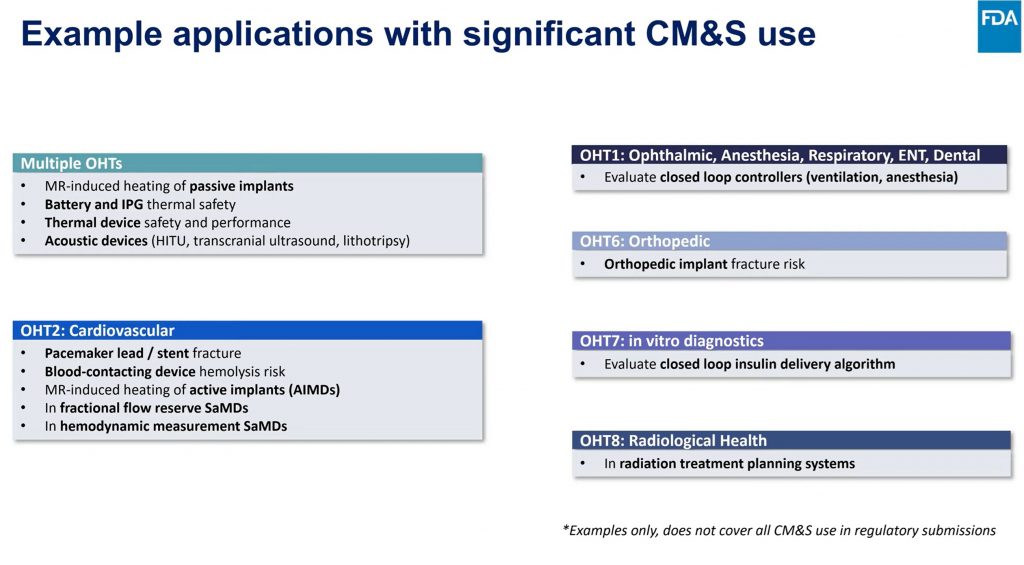
Pras Pathmanathan from the FDA’s Office of Science and Engineering Laboratories emphasized that modeling is not theoretical—it is already being used in device submissions today. The FDA has released clear frameworks: the Simulation Study Reporting Guidance, ASME V&V 40, and the Credibility Guidance (2023). These provide guardrails for how companies should validate and report their models based on the intended context of use.
Simulation is already accepted for applications like MRI heating safety, fracture risk, closed-loop control algorithms, and fractional flow reserve. While a universal playbook for full in-silico clinical trials is still evolving, FDA encourages companies to engage early through Q-Submissions to align on credibility plans and statistical strategies.
Tina Morrison, formerly Senior Executive & Science Advisor to FDA Chief Scientist at the FDA, now advising Virtonomy, added that sponsors should model not only anatomy but also physiological use conditions—for example, activity versus rest, growth and remodeling, and durability under stress. Simulation can even justify putting pediatric sizes on-label for certain adult devices, enabling regulators to track outcomes more systematically.
Industry ROI: Where to Begin
From the industry side, Parker Tyler of Edwards Lifesciences advised companies to start small. Too many digital projects fail by trying to tackle every application at once. Instead, she suggested focusing on a single high-value question—such as sizing, apposition risk, or trial cohort definition—where data and talent are already accessible.
“The key is learning,” she said. “If a simulation increases your ability to learn about risk and performance faster than traditional methods, it’s already a win.” For pediatrics, the greatest impact will likely come from class III devices for neonates and infants, where the unmet need is highest.
Key Takeaways
- The pediatric innovation gap is real and widening. Traditional methods alone cannot close it.
- In-silico tools are available now. They can reduce risk and cost across design, trials, and clinical use.
- Regulators are ready to engage. FDA has frameworks in place and encourages early conversations.
- Start small, then scale. The most successful projects begin with a focused question of interest before expanding.




