Sex equality in healthcare refers to the equal treatment and access to healthcare for both men and women. This includes ensuring that healthcare services and treatments are provided to both sexes without discrimination and that healthcare policies and programs address the specific health needs of both men and women. Even though everyone should have access to the best possible treatment despite their sex, groups such as women are still underrepresented in medical trials. Other than women, there are still other smaller population groups that face the same problem. One of our goals is to help close the disparity gap by advocating the inclusion of women in clinical trials. And one way we do that is by continuously expanding our database of female digital twins.
Why do we need trials customized to a specific sex?
Humans all vary from each other. Every person is different, has a different physique, different disease symptoms, and reactions to medications. Accordingly, surgical procedures and medical products must be adapted to different circumstances and tested under a wide variety of conditions. What works for one person may not work for another due to other pre-existing conditions or anatomy. In the healthcare industry, medical device developers often face the challenge of not being able to test on all individuals.
Some chronic diseases, such as heart disease, affect men and women differently, and healthcare providers need to be aware of these differences when diagnosing and treating patients.
This will help to improve the outcome of the treatment.
The differences between men and women are particularly evident in the example of heart attacks. Pain in one arm and in the chest are some of the symptoms that indicate a heart attack – for men. For women, other symptoms such as increased fatigue, stomach complaints or pain between the shoulder blades point to heart attacks.
Another example: The use of medication. Age, body weight, the proportion of muscle and fat tissue, and genetic differences are all decisive factors. All of these differ between persons and sexes and affect the success of the treatment.
In the medical industry, women in particular are often considered an underrepresented group. Particularly in the development of new medical products or devices, women are included less than men in clinical trials. However, it is just as important to know how the female body reacts. The risk of failure increases immensely if tests and analyses are not carried out in advance. An incorrect medication dosage or the insertion of an implant that does not fit can lead to significant health damage.
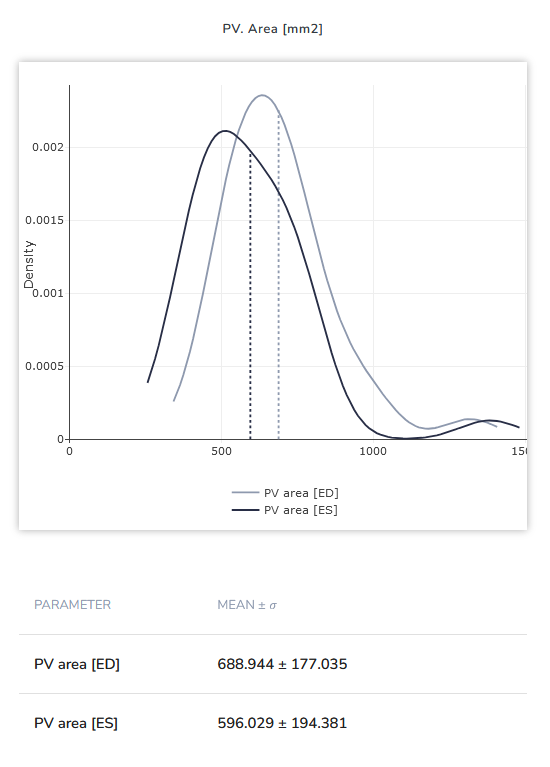
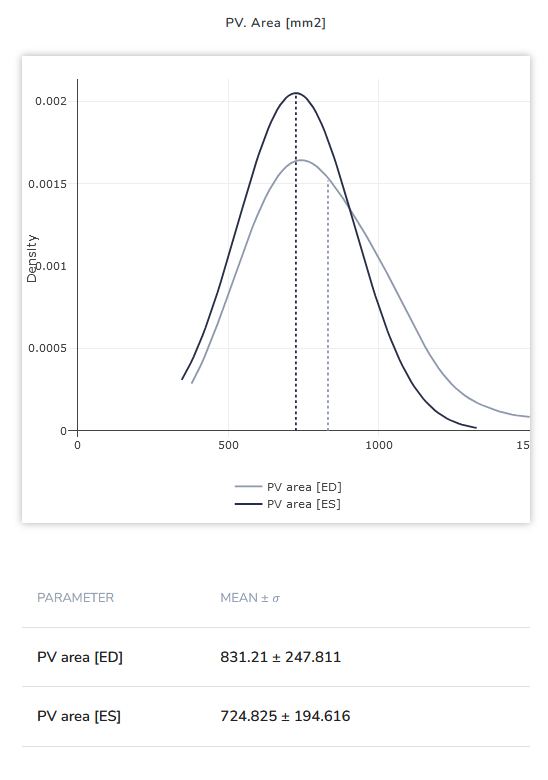
Image 1: Difference between Pulmonary Valve Orifice area (in mm²) between female (left) and male (right) patients for end-systole (ES) and end-diastole (ED) each. Statistics taken from a cohort of tricuspid valve insufficiency patients.
What are the most common reasons for the underrepresentation?
Women have historically been underrepresented in clinical trials and medical research, including in the testing of medical products. There is a number of reasons behind it, some biological and some stemming from societal factors.
1. Biological differences
Women have hormonal and physiological differences that can affect the way drugs are absorbed, distributed, and metabolized in their bodies. In addition, the female body has differences before and after menopause. Contraceptives and menstrual cycle also affect the female body and differ from woman to woman, and from time to time. Due to this very sex-specific hormonal cycle that can affect drug behavior, it is not as straightforward to perform trials as it might be with men. However, it is still important to ensure that drugs and devices are properly tested, safe, and effective for both sexes.
2. Pregnancy and breastfeeding
One of the most cited reasons for the underrepresentation of women in medical studies and testing procedures is that it is often not possible to say in advance to what extent the resulting products will have lasting effects on pregnancies or breastfeeding behavior. This is the main reason why women of childbearing age in particular are underrepresented in clinical studies.
3. Cultural and societal factors
There may be cultural and societal factors that discourage women from participating in clinical trials. For example, women may be responsible for caring for children or elderly, which can make it difficult for them to participate in clinical trials that require frequent visits to medical centers. This might also lead to institutional bias that favors male subjects in clinical trials. Some researchers may assume that male subjects are more reliable or easier to recruit than female subjects.
To address these issues, efforts are being made to increase the representation of women in clinical trials, such as setting quotas or requiring that a certain percentage of participants be women.
An example of that is the FDA’s policy from 1993 which requires inclusion of both sexes in clinical trials [1]. Since 2004, it has been mandatory in Germany to consider and investigate possible differences between women and men in clinical studies [2]. In other countries, there are often no obligations for sex equality in clinical trials.
What needs to be noted is the fact that it is not about creating a 50:50 split between men and women in the clinical trials. Rather, the goal should be to base the ratio on the actual sex distribution of diseases and to ensure that clinical trials are representative of the populations that will use the drugs or medical products being tested. Basing the proportions of male and female subjects on how often the disease actually occurs in the different biological sexes is considered a better approach.
How can female digital twins help include more women in clinical trials?
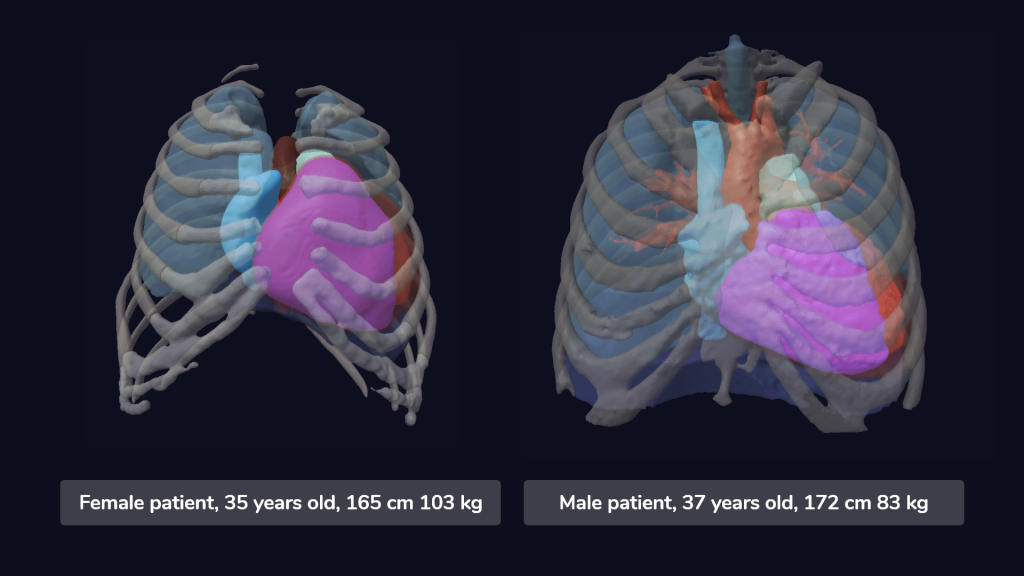
Image 2: Models of a male and female digital twin, taken from our cohort of heart failure patients.
A non-invasive method of testing on a huge number of patients
Digital twins offer the potential to create virtual replicas of organs and tissues to simulate device fit and implantation outcome. Through the immense database that Virtonomy provides, medical device developers can select and subsequently test on patients of any sex. Users of our platform can additionally select a variety of factors like age, body type, and pathology so that they can test the device fit on the necessary patient target group. With the use of female digital twins, medical device developers can make sure their device covers a wider range of users.
Times and costs saved by validating the device design and fit
Digital twins can help to reduce the time and costs associated with traditional clinical trials, which could encourage more researchers to study women’s health. With our 3D viewer where users can perform virtual implantation of their device, it is possible to validate the fit and tailor it to both male and female anatomy and ensure a higher population coverage before even performing any trials. In the long run, this can save device manufacturing costs, as well as costs related to the organization of clinical trials, since testing virtually increases the chances of successful clinical trial.
➡️ Read more about improving the medical device development cycle with digital twins in this blog post
By embracing digital twin technology, we can make strides towards more inclusive and equitable clinical trials.
And ultimately lead to better health outcomes for all people.
Would you like to increase the population coverage of your device and ensure it provides a solution for both sexes? Then contact us to schedule a demo or get more information on how you can test your device on a high number of patients. Or get in touch with us to see which female digital twins we have in our database.
[1] Guideline for the study and evaluation of gender differences in the clinical evaluation of drugs. Federal Register 1993; 58 (139): 39406-16.



